Published online Mar 27, 2022. doi: 10.4254/wjh.v14.i3.634
Peer-review started: March 31, 2021
First decision: July 27, 2021
Revised: August 1, 2021
Accepted: February 22, 2022
Article in press: February 22, 2022
Published online: March 27, 2022
Processing time: 357 Days and 17.1 Hours
Hepatic encephalopathy (HE) can be considered a result of dysregulated gut-liver-brain axis function, where cognitive impairment can be reversed or prevented by the beneficial effects induced by "gut-centric" therapies, such as the administration of nonabsorbable disaccharides, nonabsorbable antibiotics, probiotics and prebiotics.
To assess the short-term efficacy and safety of the probiotic Escherichia coli Nissle (EcN) 1917 strain compared to lactulose and rifaximin in patients with minimal/mild HE.
From January 2017 to March 2020, a total of 45 patients with HE were enrolled in this prospective, single-centre, open-label, randomized study. Participants were randomly assigned at a ratio of 1:1:1 to one of the treatment groups: The EcN group (n = 15), lactulose group (n = 15) or rifaximin group (n = 15) for a 1 mo intervention period. The main primary outcomes of the study were changes in serum ammonia and Stroop test score. The secondary outcomes were markers of a chronic systemic inflammatory response (ІL-6, ІL-8, and IFN-γ) and bacteriology of the stool flora evaluated by specialized nonculture techniques after a 1 mo intervention period.
Patients who were given rifaximin or EcN showed a more significant reduction in serum ammonia and normalization of Bifidobacteria and Lactobacilli abundance compared to the lactulose group. However, the most pronounced restoration of the symbiotic microflora was associated with EcN administration and characterized by the absence of E. coli with altered properties and pathogenic enterobacteria in patient faeces. In the primary outcome analysis, improvements in the Stroop test parameters in all intervention groups were observed. Moreover, EcN-treated patients performed 15% faster on the Stroop test than the lactulose group patients (P = 0.017). Both EcN and rifaximin produced similar significant reductions in the proinflammatory cytokines INF-γ, IL-6 and IL-8. EcN was more efficient than lactulose in reducing proinflammatory cytokine levels.
The use of the probiotic EcN strain was safe and quite efficient for HE treatment. The probiotic reduced the ammonia content and the level of serum proinflammatory cytokines, normalized the gut microbiota composition and improved the cognitive function of patients with HE. The application of the EcN strain was more effective than lactulose treatment.
Core Tip: In a prospective, single-centre, open-label, randomized study, the short-term efficacy and safety of Escherichia coli Nissle (EcN) 1917 compared to that of lactulose and rifaximin in patients with hepatic encephalopathy were evaluated. The probiotic reduced the ammonia content and the level of serum proinflammatory cytokines, normalized the gut microbiota composition and improved the cognitive functions of patients with hepatic encephalopathy. The application of the EcN strain was more effective than lactulose treatment.
- Citation: Manzhalii E, Moyseyenko V, Kondratiuk V, Molochek N, Falalyeyeva T, Kobyliak N. Effect of a specific Escherichia coli Nissle 1917 strain on minimal/mild hepatic encephalopathy treatment. World J Hepatol 2022; 14(3): 634-646
- URL: https://www.wjgnet.com/1948-5182/full/v14/i3/634.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i3.634
Non-alcoholic steatohepatitis is a major cause of liver cirrhosis and hepatocellular carcinoma; both primary indications for liver transplantation[1,2]. End-stage liver cirrhosis can lead to recurrent hepatic encephalopathy (HE). HE is a brain disorder caused by hepatocellular insufficiency and/or portosystemic shunting that manifests itself in a wide range of neurological or psychiatric disorders ranging from subclinical changes to coma[3]. HE, a challenging complication of advanced liver disease, occurs in approximately 30%-45% of patients with cirrhosis[4]. HE is classified using the West Haven criteria: Minimal (MHE), covert HE (grade I) or overt HE (OHE, grades II-IV)[5]. Numerous pathogenic factors contribute to the development of this disease[6].
Ammonia and mercaptans play a dominant role in the development of HE. Ammonia is formed from the nitrogen of nutrients in the intestine, primarily by the destruction of urea by urease, which is present in the colonial microflora[7]. Under normal conditions, ammonia is metabolized by the liver to urea, but under conditions of liver damage, urea can enter the systemic bloodstream and provoke nitrooxidative stress in the brain[8]. This process is accompanied by neurotransmission and cognitive function decline. Ammonia enhances the permeability of the blood-brain barrier by increasing the concentration of aromatic amino acids in brain tissues, in particular tryptophan, which leads to the synthesis of false neurotransmitters that replace real neurotransmitters (dopamine and norepinephrine) and thus interfere with normal neurotransmitters[9]. Decreased synthesis of physiological dopamine and norepinephrine leads to inadequate neurotransmission and HE development[10]. False neurotransmitters not only can be synthesized in the central nervous system (CNS) the intestinal microflora is also a source[11]. When liver function is impaired or if there are portosystemic shunts, neurotransmitters enter the CNS, causing HE. Subsequent studies have provided some convincing evidence of the association of HE with intestinal dysbiosis. Thus, intraperitoneal administration of liposaccharides (LPS) in a mouse model of cirrhosis was associated with induction of precoma and worsening of cytotoxic cerebral oedema[12]. Moreover, small intestinal bacterial overgrowth (SIBO) is a common and increasingly recognized disorder in cirrhosis (30% to 73%)[13,14]. One of the most important predisposing factors of SIBO is small bowel dysmotility[15]. Multiple studies have shown that the presence of SIBO is strongly linked to the pathogenesis of HE[16,17]. Therefore, HE can be considered a result of dysregulated gut-liver-brain axis function, where cognitive impairment can be reversed or prevented by the beneficial effects induced by "gut-centric" therapies such as nonabsorbable disaccharides, nonabsorbable antibiotics, probiotics, prebiotics, and faecal microbiota transplantation (FMT)[18].
The treatment of choice is nonabsorbable disaccharides, such as lactulose and lactitol, which presumably acidify the stool and eradicate toxic metabolites[19]. However, treatment with lactulose is associated with nonserious (mainly gastrointestinal) adverse events such as diarrhoea[20], and one-third of these patients with HE do not respond to this standard treatment and have refractory HE[21]. Hence, newer drugs with effective improvement in HE and better side effect profiles are still being tested.
Regarding this aspect, probiotics modulating gut microbiota, and specifically those increasing urease-free strains to target ammonia production and absorption, may be considered important therapeutic options for HE patients, particularly in scenarios of noncompliance or intolerance to lactulose[22]. Probiotics are defined as live microorganisms promoted with claims that they provide health benefits when consumed in adequate amounts[23-25]. They are considered generally safe and may bring the health benefits claimed for them[26,27]. An early meta-analysis of the effects of pre-, pro-, or synbiotics that modulate the gut microbiota showed a significant improvement in MHE[22]. However, most of the assessed probiotics were limited to Lactobacillus or Bifidobacterium strains. The probiotic strain Escherichia coli Nissle 1917 (EcN), in contrast to a number of Lactobacillus or Bifidobacterium strains, stimulates the production of the anti-inflammatory cytokine interleukin (IL)-10[28]. Given certain metabolic processes of normal microflora and the features of the EcN strain, including short-chain fatty acid (SCFA) generation, bile acid metabolism, an increase in anti-inflammatory cytokines and a decrease in proinflammatory cytokines[29], their use may be effective for the treatment of HE in cirrhotic patients.
The aim of the present study was to assess the short-term efficacy and safety of probiotic EcN strains compared to lactulose and rifaximin in patients with mild (Stage 1-2) or MHE.
This study was conducted at Bogomolets National Medical University between January 2017 and March 2020. A total of 45 patients with HE were enrolled in this prospective, single-centre, open-label, randomized study. The inclusion criteria were as follows: adult patients (age: 18-65 years) with cirrhosis diagnosed on the basis of liver biopsy, liver stiffness measurement or radiological study and the presence of minimal or mild (Grade 1-2) HE as defined by West Haven criteria; two or more documented episodes of HE in the last 6 mo, in addition to at least one episode in the last 3 mo; and a signed informed consent form. Patients were excluded if they had received L-ornithine-L-aspartate, zinc, metronidazole, neomycin, antibiotics, probiotics and yogurt consumption in the previous six weeks or if they had a history of allergy or intolerance to lactulose and/or rifaximin. The other exclusion criteria were neurologic diseases such as Alzheimer's disease, Parkinson's disease or nonhepatic metabolic encephalopathies, severe current disease (hepatic, renal, respiratory, or cardiovascular), pregnancy, any condition thought to be associated with poor compliance (e.g., alcoholism or drug addiction) or any condition or circumstance that would, in the opinion of the investigator, prevent completion of the study or interfere with analysis of study results.
This prospective, open-label, single-centre, randomized clinical study compared probiotic EcN strains with lactulose and rifaximin treatment for 1 mo in patients with mild (Stage 1-2) or MHE. The 45 participants were randomly assigned at a ratio of 1:1:1 to one of the treatment groups using a computer-generated numeric sequence. The EcN group (n = 15) received probiotics (2,5-25·109 colony forming units - CFU/g) according to the scheme for the first 4 days, 1 capsule (QD), and then twice daily (BID) for 1 mo. Participants in the lactulose group (n = 15) received 30-60 mL in 2 or 3 divided doses so that the patient passed 2-3 semisoft stools per day for 1 month of the intervention period. The third group (rifaximin group, n = 15) was prescribed oral rifaximin 500 mg two times per day.
Patient compliance was evaluated by remnant pill counting and direct questions from an investigator after completion of the treatment. Compliance was defined as good when less than 15% of the pills were unconsumed at remnant pill counting. If it was found that a participant had missed > 15% of the suggested doses, the subject data were excluded from the final results. At the same time, all of the patients were asked about adverse events (AEs). In case of minor AEs, the participants had an opportunity either to continue or to cease taking the medication but nevertheless were asked to complete further visits. Patients who reported serious AEs caused by the intervention, such as diarrhoea, nausea/vomiting or sepsis; who underwent changes in previous therapy; or who had taken antibiotics other than rifaximin were not included in the final analysis.
The study protocol was approved by the Ethics Committee at Bogomolets National Medical University (protocol number: 106/2017) and was registered in the Clinical Trial.gov database under entry number NCT04787276.
After informed consent was signed, the patients provided samples of their blood serum in a fasting state, which were immediately frozen at -20 °С. Corresponding clinical and demographic data were gathered for each patient.
The main primary outcomes of the study were changes in serum ammonia and the Stroop test after a 1 mo intervention period. Cognitive functions were determined by the Stroop test[30] using the mobile application EncephalAppStroop. Each patient took the test on a smartphone twice (before and after treatment), and all results were recorded. The test consisted of two stages: without the Stroop-off effect and with the Stroop-on effect. At each stage, patients were presented with stimuli coloured red, blue, or green, and they were required to accurately label the colour. It was necessary to identify 10 stimuli in each stage of the test, and there were 5 total iterations in each stage. Before each stage, the program issued 2 training iterations. If the patient made a mistake, (i.e., pressed the wrong colour), the iteration was stopped and rebooted from the beginning, and the patient had to complete 5 iterations without error. In the Stroop-off stage, patients saw a neutral stimulus "###" on the screen in one of three colours and had to set the colour correctly. At the Stroop stage, patients saw the text stimuli, "RED", "BLUE", and "GREEN” on the screen, and each inscription could be in three possible colours (red, blue, or green), producing a total of 9 possible combinations. The patient had to evaluate the colour of the text without errors despite the written name of the colour. The stage with the Stroop effect is more complicated because there are more errors, and more time is needed to respond when the colour is not indicated by its name (for example, the word "red" is printed in blue-coloured font instead of red-coloured font). At the end of the test, the total time(s) required to complete the Stroop-off and Stroop-on stages was estimated.
The secondary outcomes of the study that were considered for investigating the efficiency of the intervention were markers of a chronic systemic inflammatory response (ІL-6, ІL-8, and IFN-γ) and bacteriology measured in the stool flora by specialized nonculture techniques.
All patients underwent bacteriological examination of faeces for dysbiosis. The percentage of patients in each group characterized by a decrease below the normal content of symbiotic bacteria Bifidobacterium (less than 107 CFU/g), Lactobacilli (less than 107 CFU/d), E. coli with normal properties (less than 106 CFU/d) and increase in the content of E. coli with altered properties (more than 106 CFU/g), pathogenic enterobacteria (not normally detected) and Candida (more than 104 CFU/d) was determined. Given that some patients were characterized by changes in one component of the microflora and others were within normal limits, we also determined the percentage of patients characterized by changes in the content of at least one of the representatives of microbiocenosis.
The serum levels of ammonia and cytokines were determined following a 12-h fasting period by the hospital clinical laboratory. Cytokine levels were determined (IL-6, IL-8, and IFNγ) using ELISA kits from Vector Best (Novosibirsk, Russia). The concentration of cytokines was calculated according to the calibration schedule and expressed in pg/mL.
Statistical analysis was performed using the standard software SPSS version 20.0 (SPSS, Inc., Chicago, Illinois) and GraphPad Prism, version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Quantitative changes are presented as the mean and standard error (М ± SE), and qualitative changes are presented as percentages. To prove the normal distribution hypothesis, the Kolmogorov-Smirnov one-sample test was used. Data distribution was analysed using the Kolmogorov-Smirnov normality test. Variables with a parametric distribution were then analysed using one-way analysis of variance (ANOVA), and if the results were significant, a Tukey post hoc test was performed. Data with a nonparametric distribution were analysed using the Kruskal-Wallis test. To compare the data in the same patients before and after treatment, Student's t-test for dependent samples was employed. The χ-square test was used to assess differences between categorical data. Differences between groups were considered significant at a value of P < 0.05.
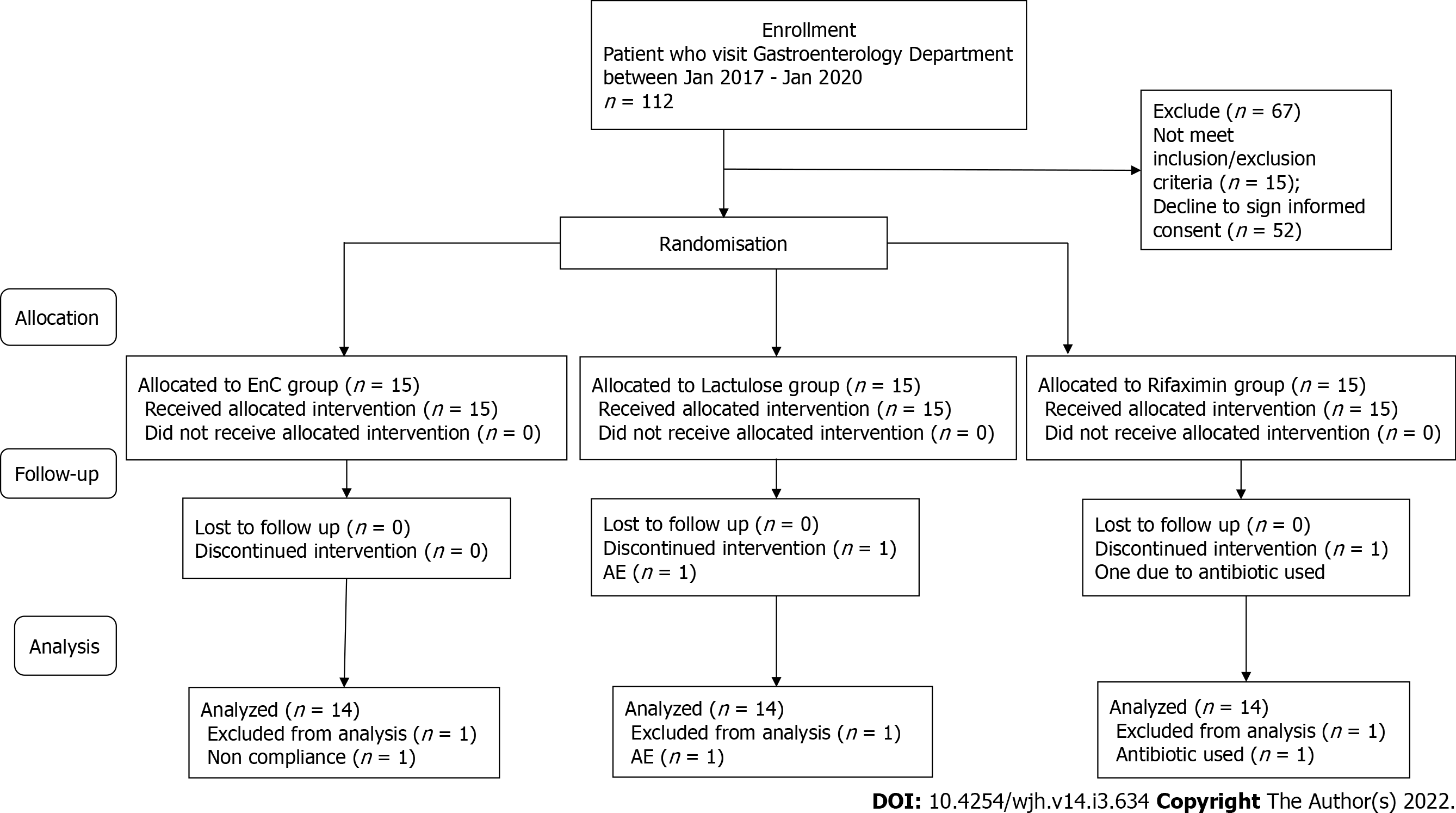
Recruitment started in January 2017 and continued until January 2020. For enrolment, the patient database of the Gastroenterology Department was used. For primary analysis, 112 patients were selected. After careful consideration for compliance with the inclusion/exclusion criteria, 15 patients were not eligible. The main reasons were the previous use of agents that can impact gut microbiota composition and overt HE (grades III-IV) as defined by West Haven criteria. A face-to-face conversation was held with all other potential participants explaining the main study criteria, purpose and methodology. After consideration of the proposal, 52 patients refused to give their informed consent. At the end of the enrolment period, with possible bias adjustment, 45 patients with HE were chosen to be included in the study. All patients were equally distributed in a random order to take the intervention for 1 mo. A CONSORT flow chart with a general protocol schedule is shown in Figure 1.
Of the 45 patients, 43 (95.5%) completed their allocated regimens. The remaining 2 patients (4.5%) were excluded from the study analysis. One patient from the lactulose group permanently discontinued participation because of diarrhoea. After AE onset, the lactulose dosage was lowered to 10 mL following 5 mL two times a day, but the event did not resolve and led to the patient’s discontinuation. Another patient from the rifaximin group had been treated with antibiotics. One patient from the EcN group was excluded from the analysis due to noncompliance, as this participant received less than 85% of the prescribed intervention. Therefore, the data from 42 (93.3%) study participants were included in the final per-protocol analysis (Figure 1).
The average patient age was 48.95 ± 6.51 years, and the HE duration ranged from 5 to 12 years. Of these patients, 33.3% of patients exhibited grade I, 26.2% exhibited grade II and 40.5% exhibited MHE according to the West Haven criteria. The baseline demographic and clinical characteristics of the enrolled patients did not significantly differ between groups (Table 1).
| Lactulose group | Rifaximin group | EcN group | P value1 | |
| Age, yr | 48.92 ± 1.64 | 49.07 ± 1.76 | 48.85 ± 1.93 | 0.996 |
| Male, % (n) | 78.6 (11) | 78.6 (11) | 71.4 (10) | 0.877 |
| Etiology of cirrhosis | ||||
| HCV, % (n) | 57.1 (8) | 42.9 (6) | 50.0 (7) | 0.940 |
| Alcoholism, % (n) | 21.4 (3) | 35.7 (5) | 28.6 (4) | |
| Mixed, % (n) | 21.4 (3) | 21.4 (3) | 21.4 (3) | |
| Cirrhosis duration, years | 8.14 ± 0.61 | 8.00 ± 0.61 | 8.07 ± 0.60 | 0.986 |
| Time to progression from hepatitis to cirrhosis, years | 4.00 ± 0.41 | 3.42 ± 0.38 | 3.56 ± 0.32 | 0.468 |
| Child-pugh score | ||||
| A, % (n) | 35.7 (5) | 42.9 (6) | 28.6 (4) | 0.733 |
| B, % (n) | 64.3 (9) | 57.1 (8) | 71.4 (10) | |
| HE grade | ||||
| MHE, % (n) | 42.9 (6) | 35.7 (5) | 42.9 (6) | 0.979 |
| Grade 1, % (n) | 35.7 (5) | 35.7 (5) | 28.6 (4) | |
| Grade 2, % (n) | 21.4 (3) | 28.6 (4) | 28.6 (4) | |
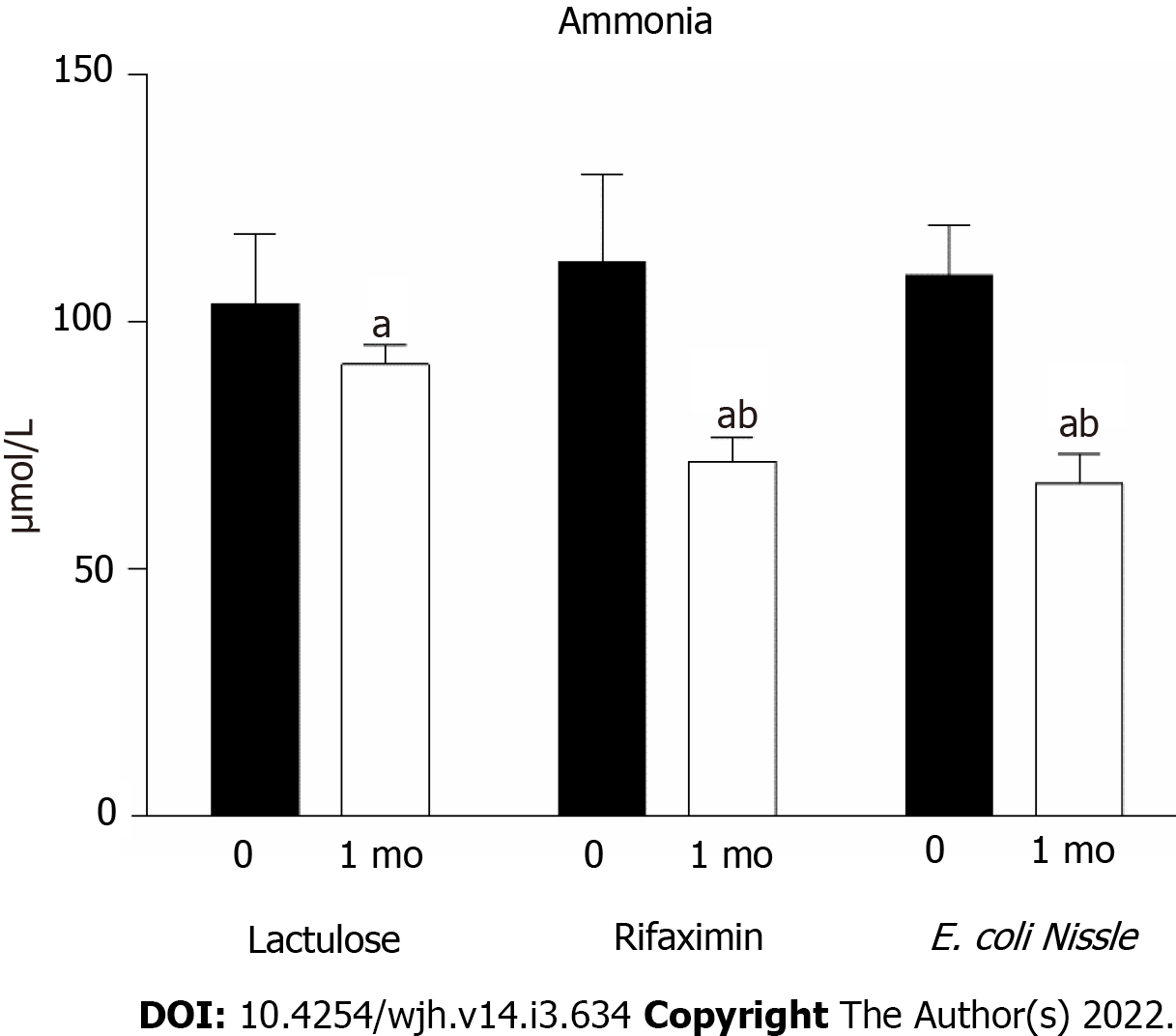
After treatment with lactulose, the concentration of ammonia decreased by 11.8% (P < 0.05) (Figure 1). Patients who were given rifaximin or the EcN probiotic strain showed a more significant reduction in ammonia than after lactulose. In the rifaximin group, the ammonia content decreased by 35.9% (P < 0.05) after treatment and by 21.5% (P < 0.05) compared to the level of ammonia in patients receiving lactulose (Figure 2). The rate of ammonia reduction in the EcN group was 38.5% (P < 0.05). Moreover, the obtained data indicate that the therapeutic use of EcN was almost 30% more effective than lactulose (Figure 2).
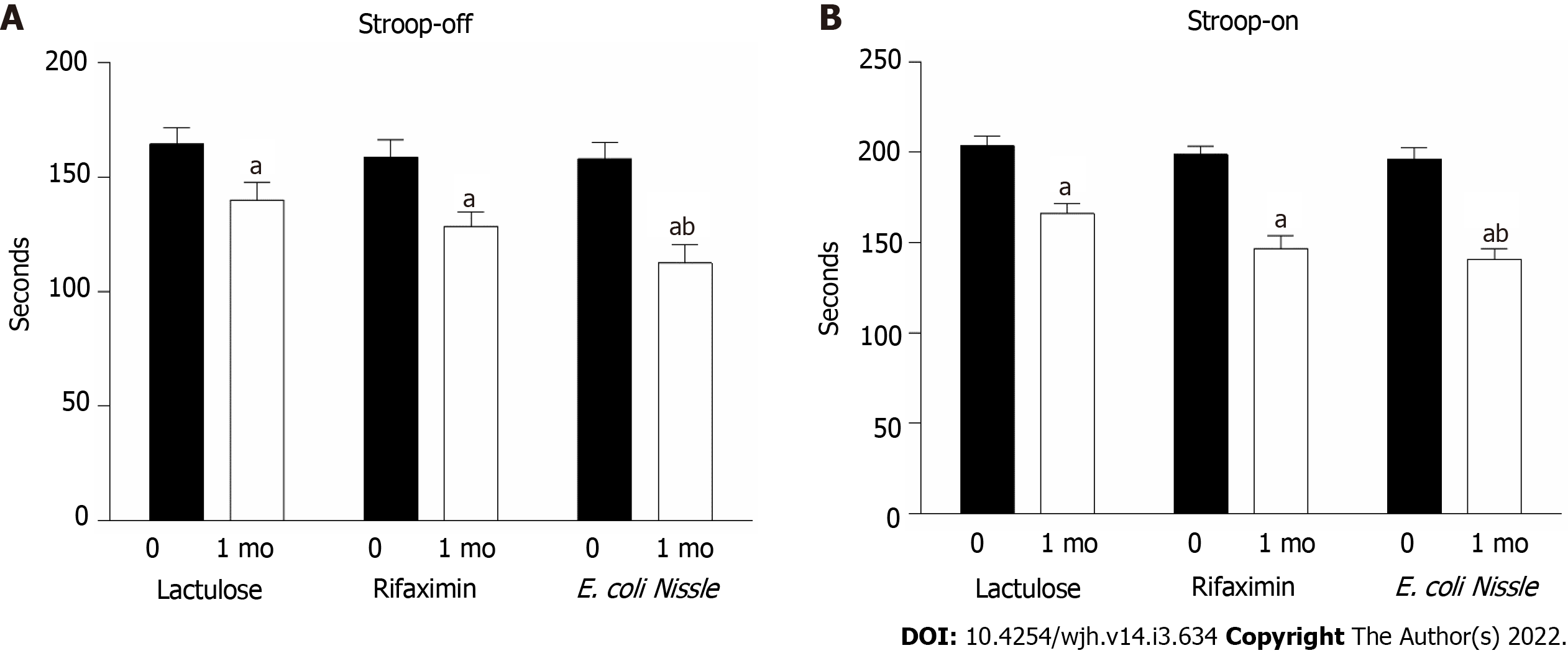
Cognitive impairment in terms of primary outcome analysis was assessed separately in patients with HE according to the Stroop test, which was divided into two stages. In the first and simpler stage (Stroop off), the mobile application was presented to patients with a text stimulus "###" in one of three possible colours (red, blue, or green), and the patient had to accurately assess the colour. The total time of correct determination of 10 presented stimuli was recorded over five iterations (i.e., the total number of responses was 50). It has been shown that the test time for patients with HE exceeded the test time of healthy people by almost 2 times, so if a healthy person correctly determined the colour of 10 text characters in an average of less than 20 s, most patients with HE needed more than 20-30 s to pass the test (the time for 5 test solutions was 160 ± 10 s, respectively).
HE treatment significantly improved patients' cognitive abilities. Under the conditions of lactulose administration, the time required to resolve the Stroop-off test was reduced by 14.9% (P = 0.028), after treatment with rifaximin by 19.0% (P = 0.001), and in EcN by 28.7% (P < 0.001). The efficiency of probiotics in restoring mental performance was higher than that of lactulose (Figure 3A).
In the second stage (Stroop on, with the Stroop effect), the program presented one of the three possible text stimuli "RED", "BLUE", "GREEN" in three possible colours (red, blue, or green), i.e., there were a total of 9 possible combinations, and the patient had to accurately assess the colour of the text regardless of its signage. The difficulty of this stage lies in the need to match the colour correctly while ignoring the name of the colour, so the total time to pass this test was slightly longer than that of the Stroop-off stage.
Patients with HE had a correct response rate 2 times lower than that of healthy people. The rate of Stroop’s test was increased in all intervention groups: For lactulose from 203.71 ± 5.33 to 166.07 ± 5.39 (P < 0.001), for rifaximin from 198.93 ± 4.43 to 146.86 ± 7.09% (P < 0.001) and for EcN from 196.43 ± 6.25 to 140.71 ± 6.07% (P < 0.001) seconds after treatment (Figure 3B). However, complete recovery of cognitive function was not recorded for all patients. It should be noted that the efficacy of the probiotic compared to lactulose was noted according to the results of the second stage. Patients who were prescribed EcN completed the test 15% faster (P = 0.017) than the lactulose group (Figure 3B).
Along with liver dysfunction, patients were diagnosed with gut dysbiotic disorders. More than 85% of patients in all groups were characterized by changes in at least one group of normoflora (Table 1). The content of Bifidobacteria and Lactobacilli was less than 107 CFU/g in more than 70% and 57% of patients, respectively (Table 1). Approximately 30% of patients had a reduced content of Escherichia coli with normal properties and an increased content of bacteria with altered properties. Pathogenic enterobacteria were detected in 35.7% of each group of patients with HE, and Candida were found in almost half of the patients (Table 2).
| Group of microflora | Percentage of patients with dysbiotic disorders, % | |||||
| Lactulose group | Rifaximin group | EcN group | ||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Bifidobacteria | 78.6 | 57.1a | 71.4 | 42.9a | 85.7 | 28.6a,b |
| Lactobacilli | 64.3 | 42.9a | 57.1 | 35.7a | 57.1 | 21.4a,b |
| E.coli with normal properties | 28.6 | 28.6 | 35.7 | 28.6 | 35.7 | 7.1a,b |
| E.coli with altered properties | 28.6 | 21.4 | 28.6 | 14.3a | 28.6 | 0.0a,b |
| Pathogenic enterobacteria | 35.7 | 28.6 | 35.7 | 21.4a | 35.7 | 0.0a,b |
| Candida | 42.9 | 35.7 | 50.0 | 28.6a | 50.0 | 7.1a,b |
| A change in at least one group of microorganisms was revealed | 85.7 | 71.4a | 92.9 | 64.3a | 92.9 | 28.6a,b |
With lactulose application, the percentage of patients with dysbiotic disorders of Bifidobacteria and Lactobacilli significantly decreased. Significant improvement of other microflora indicators in this group was not registered. In the rifaximin group, normalization of Bifidobacteria and Lactobacilli was observed in 28.6% (P < 0.05) and 21.4% (P < 0.05), respectively. There was also a decrease in the number of patients with increased levels of Escherichia coli, pathogenic enterobacteria and Candida. The most pronounced restoration of the symbiotic microflora was found in the EcN group. Normalization of Bifidobacteria abundance was registered in 57.1% (P < 0.05) of patients, and Lactobacilli was registered in 35.7% (P < 0.05). After EcN treatment, E. coli with altered properties or pathogenic enterobacteria was not detected in any of the patients, and only one patient exhibited an increase in the content of yeast-like fungi (Table 2).
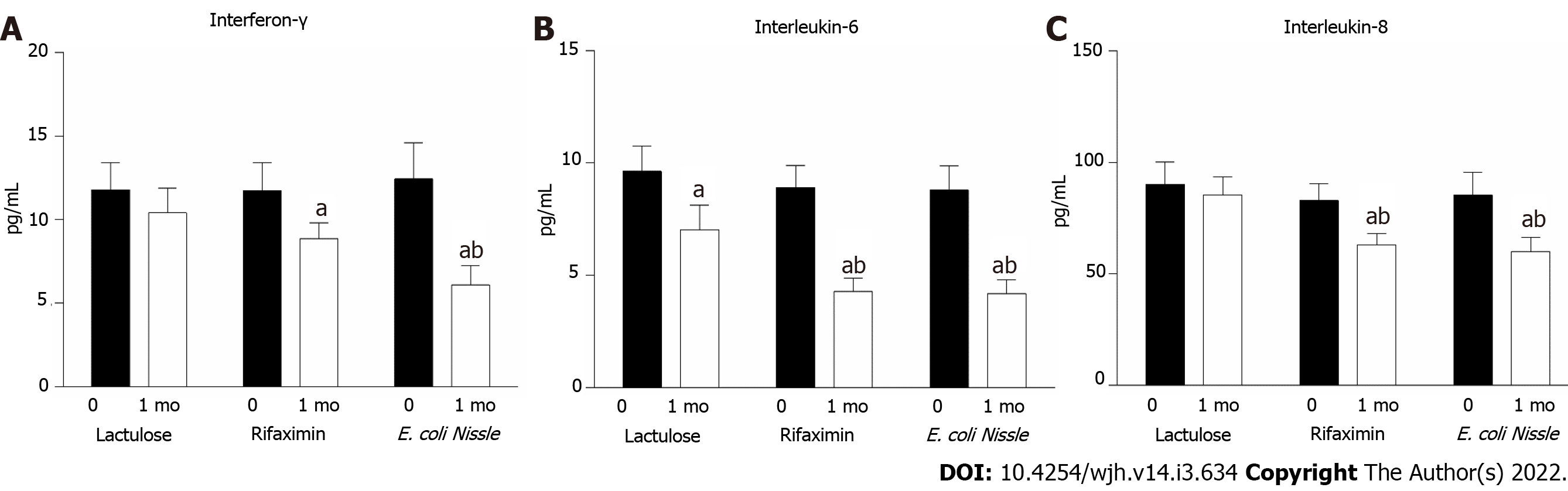
Along with liver damage, the intensification of inflammatory processes was recorded for all patients. This indicator was confirmed by an increase in the concentration of proinflammatory cytokines in the blood: IL-6, IL-8 and INF-γ were observed at frequencies 2-10 times higher than normal. For the group of patients treated with lactulose, the contents of proinflammatory INF-γ and IL-8 did not change significantly after treatment, but there was a decrease in the level of IL-6 from 9.63 ± 1.12 to 7.02 ± 1.09 (P = 0.019) pg/mL compared to the baseline level (Figure 4).
The use of rifaximin led to a significant reduction in the concentrations of serum INF-γ (11.74 ± 1.68 vs 8.86 ± 0.71 pg/mL; p=0.049), IL-6 (8.9 ± 0.98 vs 4.28 ± 0.59 pg/mL; P < 0.001) and IL-8 (82.95 ± 7.6 vs 63.02 ± 5.03 pg/mL; p=0.026) after treatment (Figure 4). For patients treated with probiotics, the reduction in inflammatory processes did not differ significantly from the effects of rifaximin. Thus, along with EcN use, the level of INF-γ decreased by 51.0% (P = 0.005), IL-6 decreased by 52.3% (P = 0.001) and IL-8 decreased by 29.6% (P = 0.007) compared to the baseline value (Figure 4). By comparing the efficacy of EcN and lactulose in the treatment of HE, one can affirm the stronger anti-inflammatory properties of the probiotic, which is 20% more efficient compared to lactulose in reducing the level of the studied proinflammatory cytokines.
There were no reported adverse events or side effects derived from the intervention across patient included to final per protocol analysis, this was evidenced by the fact that there were no changes in biochemical tests at the end of the intervention in either group.
Microbiota dysbiosis and chronic systemic inflammation are among the risk factors for the onset and progression of pathologies such as obesity, nonalcoholic fatty liver disease and liver cirrhosis[31], but changes in the intestinal microflora and inflammation in patients with HE have not been adequately studied[32]. As a result, there is an evident need to determine the impact of chronic inflammation and microflora on the epithelium of the intestinal wall, which can also affect the development of HE in people with liver disease[33]. The reduced detoxification function of the microbiota in intestinal dysbiosis increases the load on the enzymatic systems of the liver, which aggravates its metabolic and structural changes[34]. In addition, patients with liver cirrhosis showed a positive correlation between Porphyromonadaceae and Alcaligenaceae as well as low expressiveness of cognitive tests. These observations serve as additional confirmation that the increase in ammonia concentration is associated not only with liver dysfunction. Bajaj et al showed an increase in the content of ammonia-producing bacteria Alcaligenaceae in the intestine under HE conditions[35]. Successful recovery of the microflora can significantly reduce the activity of bacterial urease, absorption of ammonia in the intestine and the intensity of inflammatory processes and endotoxaemia, which is due to reduced absorption of toxins, including indoles, oxindoles, phenols and mercaptans[34]. Therefore, current strategies of HE treatment must also affect the intestinal microbiota.
Lactulose (4-O-β-galactopyranosyl-D-fructose) is widely used in the treatment of HE. It reduces pH levels in the intestine as a result of SCFA formation, creating conditions for the growth of acid-resistant Lactobacteria and Bifidobacteria that do not express the enzyme urease[7,36]. The literature regarding the effects of lactulose on the composition of microflora is quite contradictory. In contrast to reports on the restoration of indigenous microflora (Lactobacillaceae) under the influence of lactulose, Bajaj et al (2014) demonstrated intestinal dysbiosis and a decrease in the ratio between autochthonous and non-autochthonous bacteria with a high content of gram-positive bacteria Enterobacteriaceae and Bacteroidaceae despite treatment with lactulose[37].
Rifaximin is an antibiotic that is not absorbed in the gut and causes a mild change in the intestinal microflora, increasing the presence of beneficial species but without affecting the overall ratio of bacteria[38]. This modulating effect on the composition of the intestinal flora partly explains the clinical efficacy of rifaximin in reducing endotoxaemia and inflammatory markers that contribute to HE progression[39].
The data obtained in the current study show that the efficacy of lactulose as a gut microbiota recovery agent is not high enough, which is consistent with other works in which the efficacy of lactulose was not detected[40,41]. In contrast, treatment with rifaximin or EcN led to normalization of Bifidobacteria and Lactobacilli abundance. However, the most pronounced restoration of the symbiotic microflora was associated with EcN administration and characterized by the absence of E. coli with altered properties and pathogenic enterobacteria in patient features.
To our knowledge, the current study represents the first comparative analysis of the short-term efficacy of probiotic EcN strains to lactulose and rifaximin in patients with HE. One of the early RCTs failed to improve several combination tests, which showed extended reaction times in patients with MHE after treatment with EcN compared to placebo[42]. However, EcN treatment significantly improved intestinal colonization (P < 0.001) and tended to reduce endotoxin levels significantly on day 42 (P = 0.07)[42]. In contrast, our study showed the improvement of Stroop test parameters in all intervention groups after treatment. However, complete recovery of cognitive function was not recorded for all patients. Moreover, parallel with the positive shift in gut microbiota composition, patients who were prescribed EcN compared to the lactulose group completed the Stroop test 15% faster (P = 0.017).
Systemic inflammation also plays an important role in the pathogenesis of HE. Today, accumulated data suggest that the level of cytokines is not only an indicator of inflammation in chronic liver disease and PE but is a separate aetiological factor of this pathology. Systemic inflammation and neuroinflammation are communicated by peripheral tissues, which transmit signals to the brain through the activation of afferent fibres of the vagus and vascular endothelium. The blood-brain barrier (BBB) transmits signals to the brain through the formation of secondary mediators (NO and prostanoids) in response to cytokine stimulation. Cytokines increase the permeability of the BBB and directly penetrate the brain in areas of BBB disorders, where they cause the activation of microglia and the expression of proinflammatory mediator genes[43,44].
Probiotics increase anti-inflammatory cytokines and decrease proinflammatory cytokines in the blood[45]. Bacterial products have a significant effect on the intestinal-liver-brain axis as well as local and systemic immunity. Immunomodulatory activity is also indicated for SCFAs formed by the bacterial fermentation of polysaccharides. Most lactic acid bacteria in the human body are members of the genera Lactobacillus, Bifidobacterium, Propionobacterium, Streptococcus, and obligate or facultative anaerobes. These types of bacteria process carbohydrates in the intestinal lumen with the formation of SCFAs: acetic, propionic, dairy, oily, γ-oxy-oily and valerian. SCFAs play a leading role in the physiology of the large intestine, representing the main pool of anions in its lumen. SCFAs activate nerve cells by interacting with receptors associated with the G-proteins GPR41 and GPR43[46]. As recently demonstrated, SCFAs regulate the synthesis of serotonin, which is formed by enterochromaffin cells of the intestine and constitutes 95% of the body's serotonin[47]. Today, a reliable link between serotonin of intestinal origin and brain function, in particular in HE[48], may be another mechanism of communication between modulation of the microflora and disease progression.
Strain E. coli Nissle 1917, with the help of special adhesive organelles (type F-1A, F-1C and shaped fimbriae), has the ability to join the mucous membrane of the large intestine and organize microcolonies, forming biofilms[49]. They are also mobile because of the presence of flagella, which gives them the advantage of colonizing the colon. Thus, these bacteria have also been shown to enhance the mucosal barrier by interacting with immunomodulatory and anti-inflammatory mechanisms[49]. E. coli Nissle inhibits the growth of gram-negative anaerobic bacteria by secreting antimicrobial substances (microcins) and siderophores, which capture iron and thus prevent the growth of a certain pathological bacterial strain[28].
The parameters of chronic systemic inflammation in the current study were assessed in secondary outcome analysis. Both EcN and rifaximin showed similar significant reductions in the proinflammatory cytokines INF-γ, IL-6 and IL-8 compared to baseline levels. By comparing the efficacy of EcN and lactulose in the treatment of HE, one can affirm the stronger anti-inflammatory properties of the probiotic, which is 20% more efficacious than lactulose in reducing proinflammatory cytokine levels.
New research on the beneficial effects of gut microbiota modulation and related mechanisms of their interaction with liver disease should be conducted to target better a wide variety of probiotic strains. Moreover, one of the possible gut microbiota-based interventions that may be claimed in the nearest future is FMT. Preliminary data on the possible beneficial effect of FMT find support in both animal[50] and small clinical case series[51,52]. Additionally, several randomized clinical trials are actively recruiting (NCT02862249, NCT03796598, and NCT03439982) patients with HE and cirrhosis to test the efficacy of FMT.
To summarize the described results, it can be argued that the use of the probiotic EcN strain was safe and quite efficacious for HE treatment. The probiotic reduced the ammonia content and the level of serum proinflammatory cytokines, normalized the gut microbiota composition and improved the cognitive function of patients with HE. The application of the EcN strain was more efficacious than lactulose treatment.
Hepatic encephalopathy (HE) can be considered a result of dysregulated gut-liver-brain axis function, where cognitive impairment can be reversed or prevented by the beneficial effects induced by "gut-centric" therapies, such as the administration of nonabsorbable disaccharides, nonabsorbable antibiotics, probiotics and prebiotics.
The HE treatment of choice is non-absorbable disaccharides, such as lactulose and lactitol. Non-absorbable disaccharides like lactulose are associated with non-serious (mainly gastrointestinal) adverse events like diarrhea and bloating, hence, due to the side effect profile, newer drugs continue to be tested for treatment of HE. Rifaximin is an antibiotic which modulating effect on the composition of the intestinal flora partly explains the clinical efficacy in reducing endotoxaemia and inflammatory markers that contribute to HE progression. Probiotics are effective in the treatment of minimal hepatic encephalopathy. Various studies have shown some improvement in either the prevalence of minimal hepatic encephalopathy or results in neuropsychological tests with the use of probiotics.
To assess the short-term efficacy and safety of the probiotic Escherichia coli Nissle 1917 (EcN) strain compared to lactulose and rifaximin in patients with minimal/mild HE.
In total, 45 patients with HE were enrolled in this prospective, single-centre, open-label, randomized study. Participants were randomly assigned at a ratio of 1:1:1 to one of the treatment groups: the EcN group (n = 15), lactulose group (n = 15) or rifaximin group (n = 15) for a 1 mo intervention period. The main primary outcomes of the study were changes in serum ammonia and Stroop test score. The secondary outcomes were markers of a chronic systemic inflammatory response (ІL-6, ІL-8, and IFN-γ) and bacteriology of the stool flora evaluated by specialized nonculture techniques after a 1 mo intervention period.
Rifaximin or EcN showed a more significant reduction in serum ammonia and normalization of Bifidobacteria and Lactobacilli abundance compared to the lactulose group. In the primary outcome analysis, improvements in the Stroop test parameters in all intervention groups were observed. Moreover, EcN-treated patients performed 15% faster on the Stroop test than the lactulose group patients (P = 0.017). Both EcN and rifaximin produced similar significant reductions in the proinflammatory cytokines INF-γ, IL-6 and IL-8.
Probiotic EcN strain was safe and quite efficient for HE treatment. The probiotic reduced the ammonia content and the level of serum proinflammatory cytokines, normalized the gut microbiota composition and improved the cognitive function of patients with HE. The application of the EcN strain was more effective than lactulose treatment.
New research on the beneficial effects of gut microbiota modulation and related mechanisms of their interaction with liver disease should be conducted to target better a wide variety of probiotic strains. Moreover, one of the possible gut microbiota-based interventions that may be claimed in the nearest future is fecal microbiota transplantation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Ukraine
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: De Carlis R S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Kobyliak N, Abenavoli L, Falalyeyeva T, Virchenko O, Natalia B, Beregova T, Bodnar P, Spivak M. Prevention of nafld development in rats with obesity via the improvement of pro/antioxidant state by cerium dioxide nanoparticles. Clujul Medical. 2016;89:229-235. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Mykhalchyshyn G, Kobyliak N, Bodnar P. Diagnostic accuracy of acyl-ghrelin and it association with non-alcoholic fatty liver disease in type 2 diabetic patients. J Diabetes Metab Disord. 2015;14:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1392] [Article Influence: 126.5] [Reference Citation Analysis (1)] |
| 4. | Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25 Suppl 1:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | de Wit K, Schaapman JJ, Nevens F, Verbeek J, Coenen S, Cuperus FJC, Kramer M, Tjwa ETTL, Mostafavi N, Dijkgraaf MGW, van Delden OM, Beuers UHW, Coenraad MJ, Takkenberg RB. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial). BMJ Open Gastroenterol. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Bajaj JS, Lauridsen M, Tapper EB, Duarte-Rojo A, Rahimi RS, Tandon P, Shawcross DL, Thabut D, Dhiman RK, Romero-Gomez M, Sharma BC, Montagnese S. Important Unresolved Questions in the Management of Hepatic Encephalopathy: An ISHEN Consensus. Am J Gastroenterol. 2020;115:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 7. | Gómez-Hurtado I, Such J, Sanz Y, Francés R. Gut microbiota-related complications in cirrhosis. World J Gastroenterol. 2014;20:15624-15631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Shawcross DL, Wright G, Olde Damink SW, Jalan R. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis. 2007;22:125-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375:1660-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 289] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 10. | Juárez Olguín H, Calderón Guzmán D, Hernández García E, Barragán Mejía G. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxid Med Cell Longev. 2016;2016:9730467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 11. | Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203-209. [PubMed] |
| 12. | Wright G, Davies NA, Shawcross DL, Hodges SJ, Zwingmann C, Brooks HF, Mani AR, Harry D, Stadlbauer V, Zou Z, Williams R, Davies C, Moore KP, Jalan R. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology. 2007;45:1517-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther. 2009;29:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Ghosh G, Jesudian AB. Small Intestinal Bacterial Overgrowth in Patients With Cirrhosis. J Clin Exp Hepatol. 2019;9:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Eslami M, Bahar A, Hemati M, Rasouli Nejad Z, Mehranfar F, Karami S, Kobyliak NM, Yousefi B. Dietary pattern, colonic microbiota and immunometabolism interaction: new frontiers for diabetes mellitus and related disorders. Diabet Med. 2021;38:e14415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Feng Y, Cao B, Tian Q. The effect of small intestinal bacterial overgrowth on minimal hepatic encephalopathy in patients with cirrhosis. Arch Med Sci. 2016;12:592-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Campion D, Giovo I, Ponzo P, Saracco GM, Balzola F, Alessandria C. Dietary approach and gut microbiota modulation for chronic hepatic encephalopathy in cirrhosis. World J Hepatol. 2019;11:489-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 19. | Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides vs placebo/no intervention and lactulose vs lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016;CD003044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Hudson M, Schuchmann M. Long-term management of hepatic encephalopathy with lactulose and/or rifaximin: a review of the evidence. Eur J Gastroenterol Hepatol. 2019;31:434-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Leise MD, Poterucha JJ, Kamath PS, Kim WR. Management of hepatic encephalopathy in the hospital. Mayo Clin Proc. 2014;89:241-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Shukla S, Shukla A, Mehboob S, Guha S. Meta-analysis: the effects of gut flora modulation using prebiotics, probiotics and synbiotics on minimal hepatic encephalopathy. Aliment Pharmacol Ther. 2011;33:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Kobyliak N, Abenavoli L, Falalyeyeva T, Kovalchuk O, Kyriienko D, Komisarenko I. Metabolic Benefits of Probiotic Combination with Absorbent Smectite in type 2 Diabetes Patients a Randomised Controlled Trial. Rev Recent Clin Trials. 2021;16:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Molochek N, Savchuk O, Kyriienko D, Komisarenko I. Probiotic and omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance, improves glycemia and obesity parameters in individuals with type 2 diabetes: A randomised controlled trial. Obesity Medicine. 2020;19:100248. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Kobyliak N, Abenavoli L, Mykhalchyshyn G, Falalyeyeva T, Tsyryuk O, Kononenko L, Kyriienko D, Komisarenko I. Probiotics and smectite absorbent gel formulation reduce liver stiffness, transaminase and cytokine levels in NAFLD associated with type 2 diabetes: a randomized clinical study. Clinical Diabetology. 2019;8:205-214. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Eslami M, Sadrifar S, Karbalaei M, Keikha M, Kobyliak NM, Yousefi B. Importance of the Microbiota Inhibitory Mechanism on the Warburg Effect in Colorectal Cancer Cells. J Gastrointest Cancer. 2020;51:738-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Kobyliak N, Falalyeyeva T, Tsyryuk O, Eslami M, Kyriienko D, Beregova T, Ostapchenko L. New insights on strain-specific impacts of probiotics on insulin resistance: evidence from animal study. Journal of Diabetes and Metabolic Disorders. 2020;19:289-296. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 28. | Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 29. | Trebichavsky I, Splichal I, Rada V, Splichalova A. Modulation of natural immunity in the gut by Escherichia coli strain Nissle 1917. Nutr Rev. 2010;68:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Bajaj JS, Thacker LR, Heuman DM, Fuchs M, Sterling RK, Sanyal AJ, Puri P, Siddiqui MS, Stravitz RT, Bouneva I, Luketic V, Noble N, White MB, Monteith P, Unser A, Wade JB. The Stroop smartphone application is a short and valid method to screen for minimal hepatic encephalopathy. Hepatology. 2013;58:1122-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 31. | Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacology and Therapeutics. 2011;130:202-212. [RCA] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 32. | Said VJ, Garcia-Trujillo E. Beyond Lactulose: Treatment Options for Hepatic Encephalopathy. Gastroenterol Nurs. 2019;42:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 551] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 34. | Rai R, Saraswat VA, Dhiman RK. Gut microbiota: its role in hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S29-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168-G175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 414] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 36. | Gluud LL, Dam G, Borre M, Les I, Cordoba J, Marchesini G, Aagaard NK, Vilstrup H. Lactulose, rifaximin or branched chain amino acids for hepatic encephalopathy: what is the evidence? Metab Brain Dis. 2013;28:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 829] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 38. | Bajaj JS. Review article: potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Aliment Pharmacol Ther. 2016;43 Suppl 1:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 39. | Ponziani FR, Zocco MA, D'Aversa F, Pompili M, Gasbarrini A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J Gastroenterol. 2017;23:4491-4499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 40. | Riggio O, Varriale M, Testore GP, Di Rosa R, Di Rosa E, Merli M, Romiti A, Candiani C, Capocaccia L. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. Journal of clinical gastroenterology 1990;12:433-6. |
| 41. | Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004;328:1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 287] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 42. | Lata J, Juránková J, Príbramská V, Fric P, Senkyrík M, Díte P, Kroupa R. [Effect of administration of Escherichia coli Nissle (Mutaflor) on intestinal colonisation, endo-toxemia, liver function and minimal hepatic encephalopathy in patients with liver cirrhosis]. Vnitr Lek. 2006;52:215-219. [PubMed] |
| 43. | Bémeur C, Butterworth RF. Liver-brain proinflammatory signalling in acute liver failure: role in the pathogenesis of hepatic encephalopathy and brain edema. Metab Brain Dis. 2013;28:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Jayakumar AR, Rama Rao KV, Norenberg MD. Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol. 2015;5:S21-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 45. | Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 657] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 46. | Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Poulsen SS, Han S, Jones RM, Offermanns S, Schwartz TW. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552-3564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 425] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 47. | Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 2320] [Article Influence: 232.0] [Reference Citation Analysis (0)] |
| 48. | Dhanda S, Sandhir R. Role of dopaminergic and serotonergic neurotransmitters in behavioral alterations observed in rodent model of hepatic encephalopathy. Behav Brain Res. 2015;286:222-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Hancock V, Dahl M, Klemm P. Probiotic Escherichia coli strain Nissle 1917 outcompetes intestinal pathogens during biofilm formation. J Med Microbiol. 2010;59:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Wang WW, Zhang Y, Huang XB, You N, Zheng L, Li J. Fecal microbiota transplantation prevents hepatic encephalopathy in rats with carbon tetrachloride-induced acute hepatic dysfunction. World J Gastroenterol. 2017;23:6983-6994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, Kheradman R, Heuman D, Wang J, Gurry T, Williams R, Sikaroodi M, Fuchs M, Alm E, John B, Thacker LR, Riva A, Smith M, Taylor-Robinson SD, Gillevet PM. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 444] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 52. | Bajaj JS, Fagan A, Gavis EA, Kassam Z, Sikaroodi M, Gillevet PM. Long-term Outcomes of Fecal Microbiota Transplantation in Patients With Cirrhosis. Gastroenterology. 2019;156:1921-1923.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |